
Indications for Use: Cerezen™ is indicated for the treatment of mild cognitive impairment due to Alzheimer’s disease or mild Alzheimer’s disease.
Granted Designation as a Breakthrough Device by the FDA
CURRENTLY NOT APPROVED IN THE UNITED STATES

In the United States alone:
Prevalence: Over 7 Million Americans are living with Alzheimer’s Disease.
Cost: The total cost of care for people 65 and older is projected to be $384 billion in 2025.
Mortality: Alzheimer’s kills more than breast cancer and prostate cancer combined.
Caregivers: Nearly 12 million Americans provide unpaid care, contributing an estimated 19.2 billion hours of care valued at over $413 billion.
Statistics from the 2025 Alzheimer’s Association Fact Sheet.
Learn how Cerezen™ has been clinically demonstrated to improve memory and functional independence below.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

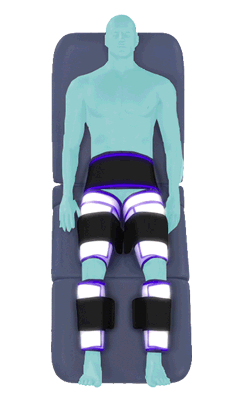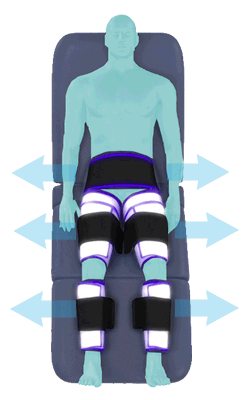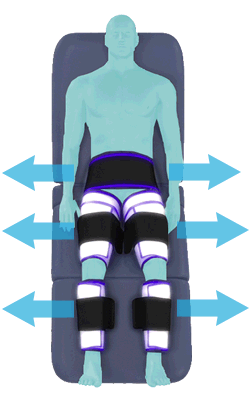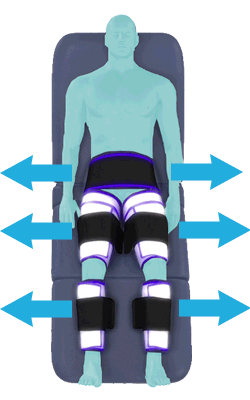
The Renew Cerezen™ system administers counterpulsation therapy. It does this by rapidly inflating/deflating air bladders contained in cloth cuffs. The cuffs are velcroed around each calf, each thigh and the buttocks/waist (a total of 5 bladders).
When inflated, the air bladders squeeze that part of the body, propelling blood from the arteries and veins in that region towards the central body / torso.
The bladders are inflated in a rapid sequence – calf to thigh to buttocks – moving blood distally to proximally.
Bladder inflation and deflation is synchronized with the patient’s ECG signal so that inflation occurs only during the diastolic portion of the heart cycle, i.e. while the heart is resting. The bladders are deflated before each heart contraction, allowing the heart to expel blood into a relatively emptied lower extremity vascular bed, reducing cardiac afterload.
A typical treatment regime consists of 35 one-hour treatments over the course of 7-12 weeks, i.e. 3-5x per week. This is typically followed by ongoing maintenance treatments twice weekly.

Inflatable air cuffs are wrapped around calves, thighs and hips.
Between heartbeats, the cuffs inflate sequentially from the calves up to the hips.
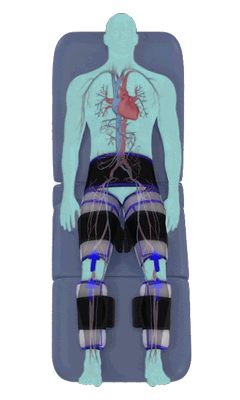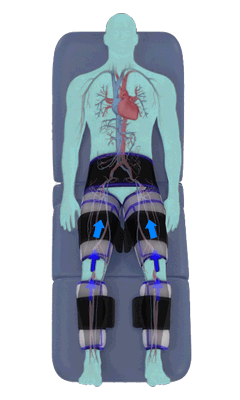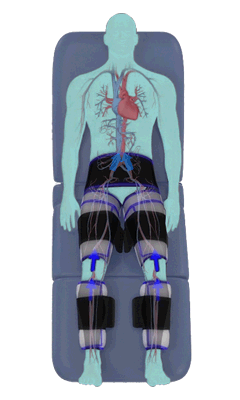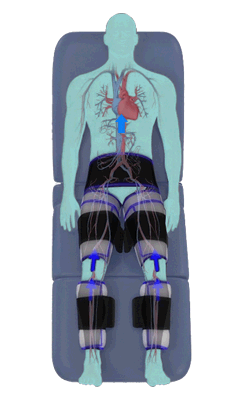
Cuff inflation compresses the blood vessels, forcing blood back to the core body. The rapid to-and-fro motion of blood is interpreted by the body as similar to exercise. This results in release of a number of chemicals such as nitric oxide which increase blood vessel diameter and reduce vascular inflammation.
The cuffs deflate simultaneously prior to the next heartbeat.
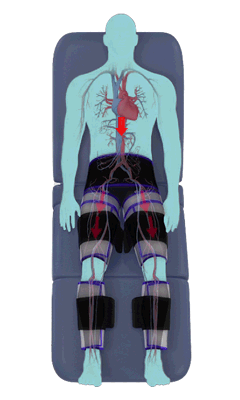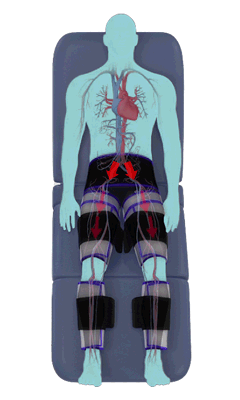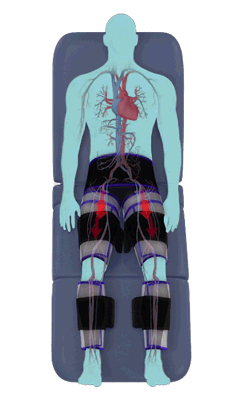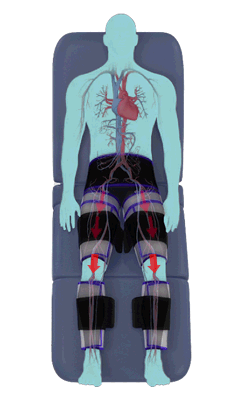
The rapid deflation of the cuffs significantly reduces the amount of work required of the heart to pump oxygenated blood to the rest of the body.
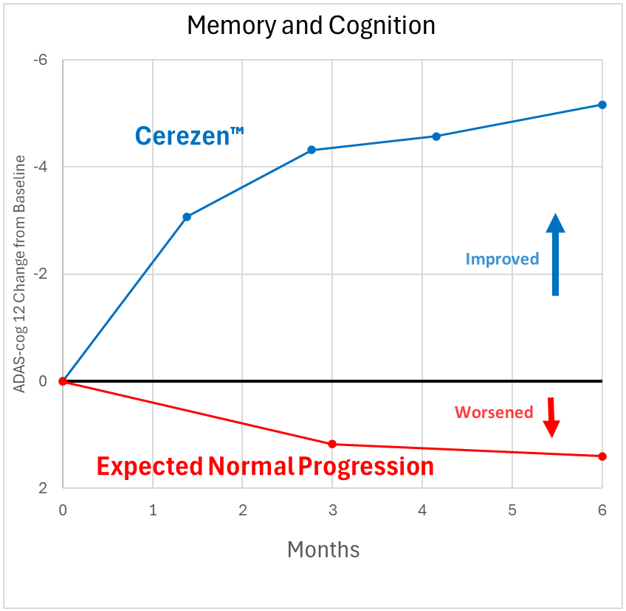
Results of pivotal trial of Cerezen™ in 190 subjects with mild cognitive impairment (MCI) or Alzheimer’s disease.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Yes, although newly certified in the EU for treatment of Alzheimer’s disease, counterpulsation technology has been used to treat cardiovascular diseases for decades with over ten million individual sessions logged. The safety record is exceptional. The most common adverse effect of Cerezen™ is chaffing of the skin under the cuffs. A review of data on over 486,000 treatments for various disorders revealed an adverse event rate of 0.007-1.3% per treatment. In a recent study of Cerezen™ treatment of 190 patients with early Alzheimer’s disease, there were no serious adverse events. This is in contrast to recent studies of anti-amyloid drugs which have found a 25-35% rate of brain swelling or bleeding (ARIA) and occasional drug-related deaths.
The concept of external counterpulsation – squeezing the legs between heartbeats to improve blood flow to the core body – was developed in the 1960s. Initially designed to help patients in heart failure, it was found to increase circulation to the heart, resulting in marked reductions in chest pain and improved exercise tolerance in angina patients. It was approved by the U.S. FDA in 1995 for treatment of heart failure and refractory angina. Subsequent research showed that counterpulsation causes improved blood throughout the body, including the brain, while also improving the ability of the arteries to increase blood flow to meet changing tissue needs.
Cerezen™ has advanced counterpulsation therapy to the point that it is now a transportable, exam-table sized device that runs from a single wall outlet. Heating, noise and other issues in older units have been eliminated resulting in comfortable, easily performed treatments.
Although much research has been devoted to treating protein buildup in the brain, 80-90+% of Alzheimer’s patients have reduced brain blood flow. In some patients this may be due to atherosclerosis (cholesterol plaques) while in others it may relate to a direct effect of the amyloid protein causing the small blood vessels of the brain to narrow. In these conditions, the brain receives insufficient oxygen and nutrients to function normally. Counterpulsation therapy has been shown to increase brain blood flow. This is hypothesized to be the one of the mechanisms of the improvement shown in Alzheimer’s patients treated with Cerezen™.
Cerezen™ has been certified in the EU under the EU MDR for treatment of mild cognitive impairment (MCI) due to Alzheimer’s disease and for early Alzheimer’s disease. Together, these disorders are referred to as early Alzheimer’s disease. If you are having symptoms of memory loss, impaired reasoning or speech, personality changes or reduced ability to function on your own, you should contact your healthcare provider for a diagnostic evaluation.
Yes. Numerous studies have demonstrated cardiovascular effects of counterpulsation therapy and many studies have shown that counterpulsation improves brain blood flow. Studies of counterpulsation therapy in Alzheimer’s disease have shown improvement in brain function. Renew conducted a study of the Cerezen™ device in treatment of 190 patients suffering from early Alzheimer’s disease. Patients received 2-5 Cerezen treatments per week for 6 months. Those receiving standard Cerezen treatments showed highly significant improvements in both cognition (reasoning and memory) and independent functioning compared to those receiving sham treatments. Detailed findings are available here.
Studies of anti-amyloid drugs have been criticized for excluding patients who had common pre-existing conditions. A Mayo Clinic study found that only 8-17% of their Alzheimer’s patients would have qualified for an anti-amyloid drug trial. On the other hand, the Cerezen™ clinical trial found that 70% of patients met criteria for inclusion and treatment. The Cerezen™ study group was 62% female, 14% Black, and 18% Hispanic.
Your healthcare provider must determine that you may benefit from Cerezen™ treatment. Once treatment is prescribed, it is administered by a trained Cerezen™ technician/operator.
Cerezen™ sessions typically last 1 hour. The usual initial treatment regimen involves 3-5 treatment sessions per week for a total of 35 treatments. After that, a maintenance regime of 2 treatments weekly is recommended.
In the latest Cerezen™ study, patients were treated for a total of 6 months. As a group, brain function continued to improve throughout that period.
Cerezen™ is a CE-marked Class IIa medical device certified under the EU Medical Device Regulation (MDR 2017/745).
Certification issued by BSI (2797), Certificate No. MDR 818385
Indication: Cerezen™ is indicated for the treatment of mild cognitive impairment due to Alzheimer’s disease or mild Alzheimer’s disease.
Intended Use: Cerezen™ is intended for use as a component in the overall management of symptoms of cognitive and/or functional impairment experienced by adults with mild cognitive impairment due to Alzheimer’s disease or mild Alzheimer’s disease. It is intended for use under the oversight of a healthcare professional.
Intended Patient Population: Cerezen™ is intended for use in adults suffering from mild cognitive impairment due to Alzheimer’s disease or mild Alzheimer’s disease.
Cerezen™ is CE-marked in the European Union. It is not cleared by the U.S. Food and Drug Administration and is not available for sale in the United States.
The following documents are for trained medical professionals only:

October 27, 2025
Cerezen™ is the first and only device certified under the new EU framework. The US FDA is a bit slower to approve it.

October 27, 2025
Cerezen’s device has shown promise in some Alzheimer’s patients and is now approved for use in Europe.

October 27, 2025
EU approval means the innovative Cerezen device could soon be available in Europe, while U.S. patients must wait for the FDA’s greenlight.
S2 Equipment LLC
26800 Haggerty Rd
Farmington Hills, MI 48380
844-209-9858
Renew Group Private Limited
463 MacPherson Road
Singapore 368181
+65-6587 7383
 Manoj Bhargava
Manoj Bhargava
Founder/CEO
Manoj Bhargava is an entrepreneur, humanitarian, and philanthropist best known as the founder of 5-hour ENERGY. A self-made billionaire, Bhargava has also launched and oversees a diverse portfolio of companies including Cerezen (Alzheimer’s treatment) and Hans Scientific (pharmaceutical contract manufacturing). His company is the largest investor in The Arena Group, a media company that operates well-known brands such as TheStreet, Parade, Men’s Journal, and Athlon Sports.
His business philosophy is rooted in practicality and purpose. Bhargava believes business should be simple, useful and focused on solving real problems. He is known for challenging conventional business wisdom and resisting trendy notions of innovation. His approach has resulted in the successful creation of not just one, but three billion-dollar companies.
Guided by the belief that wealth comes with responsibility, Bhargava has committed 99 percent of his fortune to helping others. His zero-profit business model aims to serve as many people as possible, focusing not on charity, but on empowerment through sustainable solutions. In 2009, he founded The Hans Foundation, one of India’s largest charitable endowments serving some of the world’s poorest communities.
Bhargava also started Billions in Change, a movement dedicated to developing scalable inventions that address fundamental human needs like clean water, reliable electricity and basic healthcare. His humanitarian work emphasizes enabling people to lift themselves out of poverty through access to life-changing tools and systems.
Whether in business or philanthropy, Bhargava’s north star remains the same: be useful, act with purpose, and help improve life for those who need it most.

Dr. Jack Juni
Chief Scientific Officer
Dr. Jack Juni is the Chief Scientific Officer and Medical Advisor at Stage 2 Innovations and an internationally recognized nuclear physician and inventor. With a medical degree from the University of Michigan, his career includes significant roles such as Director of Nuclear Cardiology and Director of the Positron Imaging Center at William Beaumont Hospital. Formerly an Assistant Professor at the University of Michigan Health System, Dr. Juni has over 300 publications and scientific presentations. He has also been a formidable force in research, securing grants exceeding $2.75M from prestigious organizations like the NIH and Bracco Diagnostics.
Dr. Juni holds 23 US and foreign patents and was elected a Fellow of the American College of Nuclear Physicians (FACNP). He has also served as President of the Brain Imaging Council of the Society of Nuclear Medicine and Molecular Imaging and co-authored the society’s first guidelines on brain blood flow imaging in 1997.
 Billy Tally
Billy Tally
Chief Engineer
Billy Tally brings decades of experience leading advanced engineering programs across the Automotive, Motorsports, Aerospace, Energy, Water and Medical industries. Throughout his career, he has directed the design, engineering and manufacturing of innovative, cutting-edge technologies across these diverse sectors. Bill is the inventor on multiple patents, including groundbreaking designs in the medical device and pharmaceutical fields.
 Ravi Sajwan
Ravi Sajwan
President
Mr. Sajwan’s responsibilities include providing strategic oversight and business relations of our team. A seasoned serial entrepreneur, Mr Sajwan has been involved with and/or founded four startups, each achieving successful exits.
Prior to joining our team, he co-founded Ample Communications, Inc. in 2000, where he served as Chief Technical Officer until 2008.
Before that, he was the co-founder and Chief Technical Officer of Acclaim Communications, established in 1995. His entrepreneurial journey began with the co-founding of Network Synthesis, Inc. in 1994. Mr. Sajwan held senior technical roles at StrataCom Inc. from 1989 to 1994 and served as Architect at Integrated Telecom Technology, Inc. between 1996 and 1998. He also contributed as a Chief Technical Officer to Level One Communications Inc. from 1998 to 1999, where he played a key role in shaping the company’s technical vision and long-term strategy.
Mr. Sajwan is also a director and the Chief Executive Officer of RGPL, where he has overseen the development of innovative healthcare solutions designed to improve patient care globally.
Mr. Sajwan also currently serves on the boards of LifeSignals Group, Inc., Smart Energy Systems Inc., iPaySmart Inc., iGreenTree.ai Inc., and New York University Tandon School of Engineering. Mr. Sajwan holds a Master of Science, Electrical Engineering from New York University Tandon School of Engineering (formerly Polytechnic Institute of New York, USA).
Outside of work, Mr. Sajwan enjoys mentoring entrepreneurs and is involved in many charitable entities.

Team member at vero eos et accusamus et iusto odio dignissimos ducimus qui blanditiis praesentium voluptatum deleniti atque corrupti quos dolores et quas molestias excepturi sint occaecati cupiditate non provident, similique sunt in culpa qui officia deserunt mollitia animi, id est laborum et dolorum fuga. Et harum quidem rerum facilis est et expedita distinctio. Nam libero tempore, cum soluta nobis est eligendi optio cumque nihil impedit quo minus id quod maxime placeat facere possimus, omnis voluptas assumenda est, omnis dolor repellendus. Temporibus autem quibusdam et aut officiis debitis aut rerum necessitatibus saepe eveniet ut et voluptates repudiandae sint et molestiae non recusandae. Itaque earum rerum hic tenetur a sapiente delectus, ut aut reiciendis voluptatibus maiores alias consequatur aut perferendis doloribus asperiores repellat.